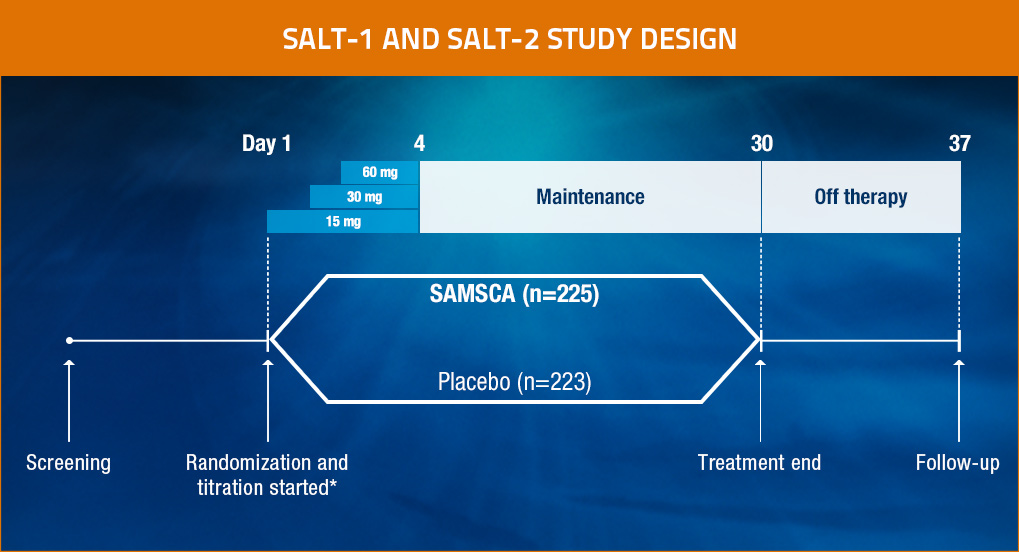
SAMSCA® (tolvaptan) Pivotal Clinical Trials Design
- The pivotal clinical trials for SAMSCA were SALT-1 and SALT-2
- SALT stands for Study of Ascending Levels of Tolvaptan in hyponatremia
- Includes patients with euvolemic or hypervolemic hyponatremia (serum sodium <135 mEq/L) due to a variety of causes, including heart failure, cirrhosis, and SIADH (SAMSCA is not indicated for patients with hyponatremia due to cirrhosis)
*Daily titration based on serum sodium levels.
Patients were evaluated at baseline, 8 hours after the first administration of study drug, and on Days 2, 3, 4, 11, 18, 25, 30, and 37. Study drugs were withheld after Day 30, and the effect of discontinuation of the study drug was assessed on Day 37.
*Daily titration based on serum sodium levels.
Objective
- To investigate whether tolvaptan, an oral vasopressin V2-receptor antagonist that promotes aquaresis—excretion of electrolyte-free water—might be of benefit in hyponatremia1
Methods
- SALT-1 and SALT-2: Two multicenter, randomized, double-blind, placebo-controlled trials (N=448)
— Identical study designs to assess reproducibility1 - Patients (aged ≥18 years) had hypervolemic or euvolemic hyponatremia (serum sodium <135 mEq/L) due to causes including heart failure (HF), cirrhosis, or SIADH1
- Patients were stratified by hyponatremia status (serum sodium 130-134 mEq/L for mild or <130 mEq/L for severe) and presence or absence of heart failure1
Primary end point
- The change in the average daily area under the curve (AUC) for the serum sodium concentration from baseline to Day 4 and from baseline to Day 301

The SAMSCA MOA video illustrates the relationship between vasopressin and hyponatremia, as well as the potential benefits of vasopressin V2-receptor antagonism.